- Loss of normal function of one of the largest human kinase proteins is at the heart of a rare and incurable genetic neurodegenerative disease known as ataxia-telangiectasia (A-T).
- The ATM (ataxia-telangiectasia mutated) gene performs many functions in all cells in our body, but its hereditary loss takes the biggest toll on important neurons in the cerebellum, leading to progressive and eventually lethally severe ataxia – disruption of the normal muscle movement control.
- Florey researchers – now supported by a Stage II US$150,000 grant, following on from the Stage I US$100,000 funding secured in 2023 – continue work on developing a next-generation treatment using antisense oligonucleotides (ASOs).
Precision medicine for ataxia-telangiectasia (A-T)
Dr Dmitry Ovchinnikov, a molecular geneticist and stem cell biologist at The Florey, has been awarded US$150,000 to validate novel precision medicines to treat a rare disease in children, ataxia-telangiectasia (A-T).
Ataxias are neurological symptoms associated with diseases often caused by neurodegenerative processes in the cerebellum, a part of the brain that controls the body’s coordination, balance and movement.
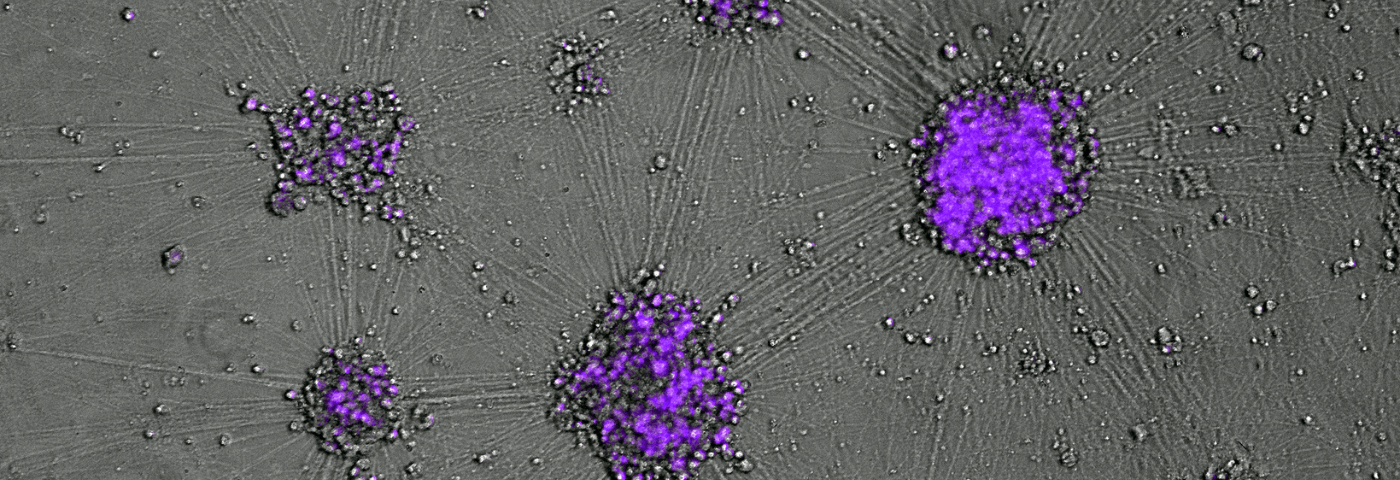
“Neurons exquisitely arranged in the folds of the cerebellum, a relatively compact brain region, account for half of all such cells found in the entire human body,” Dr Ovchinnikov says.
A-T is an incurable and fatal form of progressive neurodegeneration diagnosed in children, which affects their movement, speech and coordination, leading to severe disability and premature death. It also affects the immune system, placing children at higher risk of chest and lung infections, and increases their risk of developing various cancers.

Dr Ovchinnikov’s grant, from the main disease-focused US-based foundation A-T Children’s Project and Brisbane-based BrAshA-T, will support his research into developing techniques to increase the production and activity of the ATM gene – one of the largest genes in the human genome that is deficient or missing in children with the disease.
The ATM protein is a double-edged sword, performing multiple functions in many cells in our bodies. Too little ATM leads to ataxia, while too much could also pose some risks.
But ATM is also like a Swiss army knife, he says.
“It is one of the biggest proteins in the body that does many things in many cell types. As such, it comes with inbuilt ‘brakes’ limiting its expression. Removing the brakes allows us to achieve the desired boost by harnessing existing natural mechanisms.”
Dr Ovchinnikov says treatments like cell replacement therapy, which are currently being trialled for epilepsies or Parkinson’s disease, are not suitable for tackling A-T, due to the elaborate architecture of this brain region.
“The cerebellum’s structure is so intricate and packed that, for safety and practical reasons, we needed a different approach – to boost the activity of the affected gene.”
Dr Ovchinnikov says antisense oligonucleotides (ASOs), which offer an increase in protein expression or mRNA levels, development of which has been pioneered at the Florey by Professor Steve Petrou, may be a practical solution.
He believes the team can use their understanding of the ATM gene’s biology to boost remaining ATM in many A-T patients and ultimately, restore the gene’s function to sufficient levels.
The next stage of his research project will focus on testing the safety of efficacious ASOs, the short and stable by design DNA fragments, in human cells and in mice.
Dr Ovchinnikov thanked A-T Children’s Project and BrAshA-T for supporting his work.
“I am grateful for the international support of this project,” he said. “We hope to translate our understanding of the biology of the gene affected in this debilitating ataxia to develop effective and precise molecular therapies, with our approach designed to benefit many children affected by this disease around the world.”